Abstract
Introduction
Laser-assisted in situ keratomileusis (LASIK) exacerbates granular corneal dystrophy type 2. Post-LASIK granular corneal dystrophy type 2 is treated by several surgical techniques. To the best of our knowledge, no report has addressed the results of deep anterior lamellar keratoplasty in affected patients. Here, we report our experience regarding deep anterior lamellar keratoplasty treatment of patients with post-LASIK granular corneal dystrophy type 2.
Methods
We describe two Japanese women who underwent deep anterior lamellar keratoplasty to treat corneal opacities that worsened after LASIK.
Results
One patient had a family history of corneal dystrophies. During the initial visit to our clinic, numerous fine opacities were found at the LASIK flap interface in both patients. The clinical findings were compatible with post-LASIK granular corneal dystrophy type 2. Both patients underwent deep anterior lamellar keratoplasty by one of the authors (T.C.). In both procedures, the surgeon used a visco-dissection technique and successfully removed the whole corneal stroma. Histopathological examination of the excised corneal button from each eye revealed amyloid and hyaline deposits at the LASIK flap interface. Neither patient experienced recurrent corneal opacity during the follow-up visit at 8 years (patient 1) and 6 years (patient 2).
Conclusion
Deep anterior lamellar keratoplasty can be used for the treatment of post-LASIK granular corneal dystrophy type 2. Removal of the entire host stroma may be important for the prevention of recurrent corneal opacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laser-assisted in situ keratomileusis (LASIK) promotes protein accumulation in the corneal stroma of patients with granular corneal dystrophy (GCD) type 2, leading to severe corneal opacity. |
The surgical outcome of deep anterior lamellar keratoplasty (DALK) in patients with post-LASIK GCD type 2 has not been reported, although other surgical treatments are effective in affected patients. |
We report the relatively long-term outcomes of DALK in two patients with post-LASIK GCD type 2. |
We successfully removed the entire host stroma in both cases. DALK increased the visual acuity of both patients and prevented the recurrence of corneal opacity for 6 and 8 years after DALK. |
DALK can be used for the treatment of post-LASIK GCD type 2 without recurrence during relatively long-term follow-up. Removal of the entire host stroma may help prevent GCD recurrence. |
Introduction
Granular corneal dystrophy (GCD) type 2, also as known as Avellino corneal dystrophy or granular-lattice corneal dystrophy, is an inherited corneal disease in which one mutation causes an arginine-to-histidine amino acid substitution at codon 124 in the transforming growth factor beta-induced gene (located on chromosome 5q31). This results in amyloid and hyaline accumulation in the corneal stroma [1]. The course of GCD type 2 in heterozygous patients is generally slow and mild. However, laser-assisted in situ keratomileusis (LASIK) accelerates protein accumulation and leads to severe corneal opacities [1]. Several surgical techniques are used to treat post-LASIK GCD type 2 including opacity scraping after the flap has been lifted [2,3,4], phototherapeutic keratectomy (PTK) [3, 5], and penetrating keratoplasties [6,7,8]. However, to the best of our knowledge, no report has addressed the results of deep anterior lamellar keratoplasty (DALK) in affected patients. Here, we report our experience regarding DALK treatment of patients with post-LASIK GCD type 2.
Methods
We performed retrospective chart reviews of 3 eyes of 2 patients who underwent DALK to treat corneal opacities that worsened after LASIK. This case report was conducted in accordance with the Helsinki Declaration and was approved by the Institutional Review Board of Hiroshima University (E-709). Written informed consent was obtained from the patients for publication of this case report and any accompanying images. This manuscript includes no identifiable patient information.
Results
In case 1, a 54-year-old Japanese woman was referred for the evaluation of bilateral corneal opacities, which worsened after LASIK. She had undergone bilateral LASIK twice in another clinic and complained of blurred vision (especially in the right eye) at 7 years after the second LASIK procedure. Records of the patient’s medical history before LASIK were unavailable, and corneal opacities were not evident at the time. Notably, the patient’s father had been diagnosed with corneal dystrophy. The patient was not prescribed any systematic or topical medication. At the initial visit to our clinic, the patient’s best-corrected visual acuity (BCVA) was 20/25 OU (−2.0 −1.25 × 175 OD, −1.00 −1.00 × 175 OS). Slit-lamp examination showed numerous dense opacities at the anterior corneal stroma in both eyes, and anterior-segment optical coherence tomography detected hyperreflective materials at the surgical flap interface (Fig. 1). Except for the corneal opacities, anterior and posterior segment ocular examination was unremarkable in both eyes. These opacities were more severe in the right eye than in the left eye. The patient’s clinical findings were suggestive of post-LASIK GCD type 2. One of the authors (T.C.) performed DALK in the right eye using a visco-dissection technique [9]. The surgeon carefully removed the entire host stroma and confirmed no remnant stroma on the Descemet membrane. Histopathological examination of the excised corneal button revealed amyloid deposits (stained with Congo red) and hyaline deposits (stained with Masson’s trichrome) at the LASIK interface (Fig. 2). Postoperative medication included topical moxifloxacin hydrochloride (VIGAMOX® [moxifloxacin HCl ophthalmic solution] 0.5%, Novartis Pharmaceuticals, Basel, Switzerland) four times a day and topical betamethasone sodium phosphate (Rinderon®, Shionogi Pharmaceuticals, Osaka, Japan) four times a day. The patient’s postoperative course was uneventful, and the patient’s blurred vision was alleviated after surgery. The patient did not experience corneal stromal rejection and the graft remained clear. We removed the suture 2 years after the surgery, and the patient’s BCVA improved to 20/16 OD (−1.50) at the time. The patient’s BCVA remained stable during 8 years of follow-up. The patient did not experience recurrent corneal opacity during the follow-up period (Fig. 1).
Preoperative and postoperative clinical findings in a patient with post-LASIK granular corneal dystrophy type 2 (case 1). Preoperative slit-lamp photograph captured by sclerotic scatter and anterior segment optical coherence tomography of the right eye in case 1. The images show numerous and dense corneal opacities occupying over two-thirds of the pupillary area (a) and hyperreflective materials at the surgical flap interface (arrowheads) (b). Slit-lamp photography and anterior segment optical coherence tomography of the same eye at 8 years after deep anterior lamellar keratoplasty. The images show a clear graft without recurrent corneal opacities (c, d)
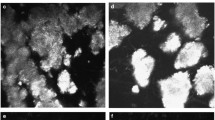
Histopathological findings in a corneal button from a patient with post-LASIK granular corneal dystrophy type 2. Histopathological analysis of tissues from case 1 revealed eosinophilic deposits along the LASIK flap interface (a, hematoxylin–eosin; original magnification ×100). Hyaline deposits were intensely stained with Masson’s trichrome (b, Masson’s trichrome; original magnification ×400). Amyloid deposits were weakly stained with Congo red (arrowheads) (c, Congo red; original magnification ×400)
In case 2, a 32-year-old Japanese woman complained of bilateral vision reduction. Five years earlier, the patient had undergone bilateral LASIK in another clinic. Corneal opacities had not been evident before LASIK. The patient’s family history did not include any inherited corneal diseases. She was not prescribed any systematic or topical medication. Similar findings as those in case 1 were observed in both eyes during the initial visit to our clinic (Fig. 3). The patient was not aware of any concomitant eye disease. The corneal opacities were denser in the left eye than in the right eye at the initial visit to our clinic. The patient’s BCVA was 20/25 OD (not correctable) and 20/50 OS (−0.5). The same surgeon (T.C.) as for case 1 performed DALK in the left eye. DALK was also performed in the right eye 2 years later because the patient’s BCVA in that eye subsequently decreased to 20/50 (not correctable), while BCVA of the left eye increased to 20/20 (not correctable) after DALK. Similar to case 1, amyloid and hyaline deposits were detected at the LASIK interface in the corneal buttons collected from both eyes. Postoperative medication included topical levofloxacin hydrochloride (Cravit® ophthalmic solution 0.5%, Santen Pharmaceuticals, Osaka, Japan) four times a day and topical betamethasone sodium phosphate (Rinderon®, Shionogi Pharmaceuticals) four times a day. The patient’s postoperative course was uneventful. We removed the suture in the right eye 1 year after the surgery. The patient did not experience corneal stromal rejection and the graft remained clear in both eyes. The patient’s BCVA was 20/16 OD (+2.75) and 20/12.5 OS (+2) at the last visit, corresponding to 4 and 6 years after the surgeries, respectively. She did not experience recurrent corneal opacity during the follow-up period (Fig. 3).
Preoperative and postoperative slit-lamp photographs of case 2. Dense corneal opacities occupy almost the entire pupil area in both eyes before deep anterior lamellar keratoplasty (a: the right eye, b: the left eye). In both eyes, the corneal opacities were successfully removed by deep anterior lamellar keratoplasty, and the patient did not experience recurrence of corneal opacities through the follow-up period (c: the right eye, 4 years after the surgery, d: the left eye, 6 years after the surgery)
Discussion
To the best of our knowledge, this case series is the first report of the long-term outcomes of DALK in eyes with post-LASIK GCD type 2. Importantly, no GCD recurrences were observed during 4 to 8 years of follow-up after DALK. Although there have been several reports of other surgical treatments for post-LASIK GCD type 2 [2,3,4,5,6,7,8], their long-term outcomes are poorly understood and only two relevant reports have been published [3, 5]. Jun et al. performed PTK in one patient with post-LASIK GCD type 2, and they scraped the interface deposits after lifting the LASIK flap in another affected patient. Notably, scraping deposits after lifting the flap resulted in a clinically significant recurrence with decreased visual acuity at 16 months. In contrast, corneal opacities did not recur in the patient treated with PTK, but the follow-up period was limited to 6 months after surgery [3]. In the other published report, PTK with LASIK flap removal yielded a lower recurrence rate than did flap-conserving PTK. Notably, subtle recurrence occurred in the flap removal group, although this did not affect visual acuity [5].
Because keratocytes in the corneal stroma may be an important source of recurrent corneal opacity in patients with GCD types 1 and 2 [10], we recommend that surgeons carefully remove the entire host corneal stroma during the treatment of patients with LASIK-aggravated GCD. This recommendation is consistent with a report by Oke et al. that involved DALK with the big-bubble technique on both corneas of a patient with GCD. In the right eye, the bubble was evident in the stromal layer, suggesting that host stroma remained on the Descemet membrane. In contrast, the surgeon cleaved a plane between the Descemet membrane and corneal stroma in the left eye. Corneal opacities recurred only in the right eye at 6 years after surgery [10]. In our patient, exposure of the Descemet membrane without preservation of the corneal stroma presumably prevented recurrent corneal opacity during long-term follow-up. Considering that the mean duration of GCD recurrence after DALK in the literature was 2.4 years (19 cases, range: 0.5 to 8.5 years) [10], our follow-up duration might be sufficient.
The histological examination of our patients showed Masson’s trichrome-positive and Congo red-positive deposits, corresponding to hyaline and amyloid accumulation. Four previous reports have described Masson’s trichrome and Congo red staining results in specimens from patients with LASIK-aggravated GCD type 2. Three of these reports showed that deposits at the LASIK interface consisted of hyaline stained with Masson’s trichrome, whereas amyloid stained with Congo red was not detected; the remaining report described hyaline and amyloid deposits at the LASIK flap [4, 6,7,8].
A notable limitation in this case report is that neither patient underwent genetic analysis. Nevertheless, amyloid and hyaline deposits in the patients’ corneal specimens and clinical findings were characteristic of GCD type 2 [1], strongly supporting the diagnosis for each patient.
Conclusions
Our report demonstrates that DALK can be used for the treatment of patients with post-LASIK GCD type 2; our patients did not exhibit recurrence during long-term follow-up. Removal of the entire host stroma may help prevent recurrent corneal opacities.
References
Weiss JS, Møller HU, Lisch W, Kinoshita S, Busin M, Aldave AJ, et al. The IC3D classification of the corneal dystrophies. Cornea. 2008;27(Suppl 2):S1-42.
Xiu HW, Hyun CL, Stulting RD, Kim T, Seung EJ, Moon JK, et al. Exacerbation of Avellino corneal dystrophy after laser in situ keratomileusis. Cornea. 2002;21:223–6.
Jun RM, Tchah H, Kim TI, Stulting RD, Jung SE, Seo KY, et al. Avellino corneal dystrophy after LASIK. Ophthalmology. 2004;111:463–8.
Lee WB, Himmel KS, Hamilton SM, Zhao XC, Yee RW, Kang SJ, et al. Excimer laser exacerbation of Avellino corneal dystrophy. J Cataract Refract Surg. 2007;33:133–8.
Jun I, Jung JW, Joon Choi Y, Kim T im, Yul Seo K, Kweon Kim E. Long-term Clinical Outcomes of Phototherapeutic Keratectomy in Corneas With Granular Corneal Dystrophy Type 2 Exacerbated After LASIK. J Refract Surg. 2018;34:132–9.
Awwad ST, Di Pascuale MA, Hogan RN, Forstot SL, McCulley JP, Cavanagh HD. Avellino corneal dystrophy worsening after laser in situ keratomileusis: further clinicopathologic observations and proposed pathogenesis. Am J Ophthalmol. 2008;145:656–61.
Aldave AJ, Sonmez B, Forstot SL, Rayner SA, Yellore VS, Glasgow BJ. A clinical and histopathologic examination of accelerated tgfbip deposition after LASIK in combined granular-lattice corneal dystrophy. Am J Ophthalmol. 2007;143:416–9.
Chiu EK, Lin AY, Folberg R, Saidel M. Avellino dystrophy in a patient after laser-assisted in situ keratomileusis surgery manifesting as granular dystrophy. Arch Ophthalmol. 2007;125:703–5.
Melles GRJ, Remeijer L, Geerards AJM, Beekhuis WH. A quick surgical technique for deep, anterior lamellar keratoplasty using visco-dissection. Cornea. 2000;19:427–32.
Oke I, Haddad N, Lee HJ. Granular corneal dystrophy recurrence at the posterior graft-host interface after type 1 big bubble deep anterior lamellar keratoplasty. Am J Ophthalmol Case Reports. 2020;20 September.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The corresponding author (Taiichiro Chikama) will be funding the journal’s Rapid Service Fee.
Medical Writing, Editorial, and Other Assistance
We thank J. Ludovic Croxford, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Conceptualization: Taiichiro Chikama, Methodology: Taiichiro Chikama, Formal analysis and investigation: Koichiro Shinji, Writing—original draft preparation: Koichiro Shinji, Writing—review and editing: Taiichiro Chikama, Funding acquisition: not applicable, Resources: Taiichiro Chikama, Sachiko Maruoka, Supervision: Yoshiaki Kiuchi.
Disclosures
The authors: Koichiro Shinji, Taiichiro Chikama, Sachiko Maruoka, and Yoshiaki Kiuchi, have nothing to disclose.
Compliance with Ethics Guidelines
This case report was conducted in accordance with the Helsinki Declaration, and was approved by the Institutional Review Board of Hiroshima University (E-709). Written informed consent was obtained from the patients for publication of this case report and any accompanying images. This manuscript includes no identifiable patient information.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shinji, K., Chikama, T., Maruoka, S. et al. Long-Term Observation of Deep Anterior Lamellar Keratoplasty in Patients with Post-LASIK Granular Corneal Dystrophy Type 2: Two Case Reports. Ophthalmol Ther 10, 1163–1169 (2021). https://doi.org/10.1007/s40123-021-00399-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-021-00399-2